Background: Cancer-related fatigue (CRF) is the most common symptom experienced by cancer patients, and it may persist for years after treatment. CRF is a stressor that often results in poor quality of life (QOL). Although previous studies found exercise to be an effective intervention for CRF, managing this particular stressor continues to be a challenge.
Objectives: The purpose of this study was to evaluate the effects of a physician-referred exercise program (PREP) on self-reported CRF and QOL in early cancer survivors.
Methods: Seventy cancer survivors were recruited using convenience sampling to participate in this quasi-experimental study. The Brief Fatigue Inventory scale was used to measure CRF and the European Organisation for Research and Treatment of Cancer QOL Questionnaire was used to measure QOL. Paired t-tests, adjusted for multiplicity, were used to examine differences between pre-PREP and post-PREP scores. The Neuman Systems Model served as the conceptual framework for this study.
Results: A total of 38 participants completed the PREP. Global CRF scores significantly decreased after the PREP with a mean paired difference of 1.99 (95% confidence interval [CI], 1.19 to 2.77; t(37) = 5.11; P = .0001; d = 0.83). QOL scores significantly increased after the PREP with a mean paired difference of -12.28 (95% CI, -19.39 to -5.17; t(37) = -3.50; P = .0012; d = 0.57).
Conclusions: Findings suggest the PREP was effective in reducing CRF and improving QOL in early cancer survivors. These benefits were found to be both statistically significant and clinically meaningful. Oncology nurses should encourage cancer survivors to engage in regular exercise in the early posttreatment period.
The number of Americans living with a history of cancer is growing as a result of improved survival rates and the aging US population. As of January 2014, there were nearly 14.5 million Americans living with a history of cancer.1 The population of cancer survivors in the US is projected to increase to 18 million by the year 2020.2 Although medical advances are affording cancer survivors increased longevity, it also means that they are living longer with the distressing physical and psychosocial side effects of cancer and its treatment.3,4
Cancer-related fatigue (CRF) is one of the most common distressing symptoms experienced by patients with cancer.5-8 The National Comprehensive Cancer Network (NCCN) defines CRF as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”9 Prevalence rates of CRF vary depending on stage of disease and treatment, but are commonly reported to be >60%, and as high as 96% for patients with cancer who are undergoing treatment.7,8 Furthermore, as many as 30% of cancer survivors report experiencing the negative effects of CRF as long as 3 years after completing treatment.8
CRF has been associated with impairment of physical, psychological, functional, and cognitive abilities, which often leads to a reduced overall quality of life (QOL).6,10,11 CRF may interfere with the patient’s normal routine when they are unable to perform activities of daily living (ADLs), concentrate, solve problems, and make decisions.12 Despite its frequency and negative impact on patients’ QOL, CRF remains underreported, underdiagnosed, and undermanaged.11,13
Exercise is a readily accessible, safe, and inexpensive intervention that has been proven effective in the management of CRF. Several meta-analyses have confirmed the benefits of exercise in reducing CRF in patients undergoing active treatment, and in cancer survivors.14-16
The Oncology Nursing Society and NCCN both recommend exercise as an intervention to reduce CRF in patients with cancer on active treatment, posttreatment, and at the end of life.9,17 Current NCCN Clinical Practice Guidelines recommend that exercise programs for patients with cancer begin with low intensity, be increased slowly, and be individualized to the patient’s condition.9 Similarly, the American College of Sports Medicine (ACSM) recommends exercise guidelines for all patients with cancer, not only to reduce CRF, but also to improve QOL and body image.18,19
The purpose of this study was to evaluate the effectiveness of a supervised, 60-day, physician-referred exercise program (PREP) on self-reported CRF and QOL in early cancer survivors.
Theoretical Framework
The Neuman Systems Model was used as a conceptual framework to guide this study.20 The model is holistic and wellness-oriented. The philosophical basis of the model encompasses the patient being comprised of 5 variables of self, which include the physiological, psychological, sociocultural, developmental, and spiritual variables. In this model, the patient attempts to reduce possible harm from internal and external stressors. The usual level of health is represented by the normal line of defense, and is protected by a flexible line of defense. When stressors break through the flexible line of defense, the system is invaded and lines of resistance are activated, which moves the system toward illness on the wellness–illness continuum.20 Primary, secondary, and tertiary prevention activities are implemented to strengthen the patient’s lines of resistance and flexible lines of defense in the presence of a stressor.20 For this study, CRF was identified as an internal stressor that can weaken the body’s flexible lines of defense, thus having the potential to negatively impact all 5 variables of self. The PREP was conceptualized as a tertiary intervention to promote health and improve QOL by reducing CRF and strengthening the patient’s flexible lines of defense.
Methods
Study Design
This quasi-experimental study used a prospective, pretest, posttest, intervention-group–only design to evaluate the effectiveness of a 60-day, supervised PREP in reducing self-reported CRF and improving QOL among early cancer survivors.
Sample and Setting
Cancer survivors who completed treatment were recruited using convenience sampling techniques between fall 2011 and spring 2014. The cancer center in which the study participants were treated is accredited by the American College of Surgeons Commission on Cancer as a comprehensive community cancer and breast center.21 Eligible patients were aged ≥18 years; diagnosed with any solid or hematologic cancer; completed chemotherapy and/or radiation therapy within the preceding 90 days; reported experiencing CRF (≥4 on a 0-10 numeric fatigue scale); and received medical clearance from the treating oncologist (ie, a signed PREP referral form). Patients receiving active cancer treatment—with the exception of oral hormonal therapy—were excluded from this study. This study was approved by the hospital’s Institutional Review Board. Verbal and written informed consent was obtained after all possible risks, benefits, and alternatives to participation were explained to each cancer survivor.
Procedures
Upon study enrollment, cancer survivors completed 2 baseline surveys, including the Brief Fatigue Inventory (BFI) Scale and the European Organisation for Research and Treatment of Cancer QOL Questionnaire (EORTC QLQ-C30).22,23 Participants then completed the 60-day, supervised PREP, which was tailored to the cancer survivor’s abilities and exercise goals. Within 14 days of completion of the PREP, the participants met with the primary researcher, and were again asked to complete the BFI and EORTC QLQ-C30 surveys.
Intervention
The 60-day PREP was based on the ACSM and American Medical Association’s “Exercise is Medicine” campaign.24 The 60-day PREP began with an initial assessment with 1 of 2 certified exercise fitness specialists at the Fitness Connection (a Medical Fitness Association–certified facility). All fitness specialists working with the study participants were certified by ACSM in conjunction with the American Cancer Society (ACS) as certified Cancer Exercise Trainers (CETs).25 The initial appointment lasted approximately 1 hour, and included a health history, review of medications, and participant education regarding target heart rates, normal blood pressure ranges, as well as an explanation and demonstration of gym equipment. In addition, mid- (30-day) and end-point (60-day) assessments were completed by fitness specialists to update health histories, assess heart rate and blood pressure, review exercise progress and goals, and provide education as needed.
The PREP protocol consisted of 2 30-minute weekly sessions. The exercise sessions included low-to-moderate–impact cardiovascular exercise (eg, walking on a treadmill or using a seated elliptical and recumbent bicycle), low-to-moderate–impact strength training exercise using stationary machines with internal band resistance (eg, leg extensions, leg curls, tricep extensions, chest presses, bicep curls, seated rowing, lateral pull-downs, abdominal crunches), and static flexibility (eg, using TechnoGym posterior and anterior stretching equipment and Precor stretching equipment). Specific exercise frequency and intensity varied according to patients’ abilities and goals. Generally, patients completed ≥30 minutes of cardiovascular exercise, followed by 3 sets of 10 repetitions of each strength training exercise at an appropriate intensity, and finished their workouts with static flexibility.
Measures
The BFI was used to assess patient-reported CRF in this study.22 This widely used scale consists of 9 items measured on an 11-point Likert scale (0-10) that assessed the severity of CRF (3 items) and the amount of interference that CRF had on functioning (6 items) in the past 24 hours. The BFI provides 3 subscale scores: (1) CRF severity, (2) CRF interference, and (3) global CRF (severity and interference). Higher scores are equivalent to higher CRF severity or interference. The BFI scores have demonstrated high reliability (Cronbach’s α = 0.96) and validity in various cancer populations.22
The EORTC QLQ-C3023 was used to measure patient-reported QOL in this study. The EORTC QLQ-C30 consists of 30 items measured on a 4-point Likert scale (1 = not at all, 4 = very much). This well-established instrument consists of a global health status/QOL scale, 5 functional scales (physical, cognitive, emotional, role, and social functioning), and 9 single-item symptom scales. Scores on the global health status/QOL and functional scales ranged from 0 to 100, with higher scores signifying better QOL and higher functioning. Multi-item scales on the EORTC QLQ-C30 were found to be reliable (Cronbach’s α = 0.52-0.89) and valid in many diverse cancer populations.23,26
Data Analysis
All data were analyzed using SPSS version 22.0. Descriptive statistics were used to summarize demographic and clinical variables. Chi-square (categorical variables) and independent t tests (continuous variables) were used to examine the differences among demographic and clinical variables between participants who completed and did not complete the PREP in this study. Paired t tests were used to examine differences among pre- and post-PREP BFI (severity, interference, global CRF), EORTC QLQ-C30 QOL (global health status/QOL), and functional scores (physical, cognitive, emotional, role, and social functioning). Post-hoc Bonferroni corrections were applied to adjust for multiple comparisons and control of type I errors across the 2 groups of study comparisons. As such, for BFI score (severity, interference, and global CRF) comparisons, a P value of ≤.017 (.05/3) was required to achieve statistical significance, and for EORTC QLQ-C30 QOL and functional status score comparisons, a P value of ≤.008 (.05/6 comparisons) was required to achieve statistical significance. Effect sizes (Cohen’s d statistic) were also calculated by dividing the mean difference of pre- and post-PREP scores by the standard deviation of the difference of the scores.
Results
A total of 70 cancer survivors met the study inclusion criteria and agreed to participate in the study, and 54.3% of them completed the PREP (N = 38). In the 32 patients who did not complete the PREP, the reasons for noncompletion included personal reasons (2, 6.3%); transportation issues (2, 6.3%); having moved out of the area (3, 9.2%); non–cancer-related illnesses (6, 18.8%); cancer-related illnesses (7, 21.9%); and being lost to follow-up (12, 37.5%). There were no significant differences in demographic and clinical characteristics among cancer survivors who completed and did not complete the PREP (all P values >.05; results not shown).
As shown in Table 1, most cancer survivors in this study were white (81.6%), non-Hispanic/Latino (97.4%), and women (71.1%), with a mean age of 65 years (Mdn = 68; range, 41-88). The most common cancer diagnoses in this sample included breast (47.4%), lung (10.5%), colorectal (10.5%), prostate (7.9%), head and neck (7.9%), and lymphoma (7.9%). According to the ACS, 46% of all cancer survivors in the United States are aged ≥70 years,1 with approximately 50.5% of all cancer survivors being women. In this study, the majority (71.1%) of cancer survivors were women, with 33% of cancer survivors aged ≥70 years. As such, our sample did not reflect the greater population of cancer survivors in the United States in terms of sex and the proportion of adults aged ≥70 years, which limits the generalizability of our study findings. However, our sample was consistent with previous exercise interventional research, which predominantly included cancer survivors who are white women.4,27
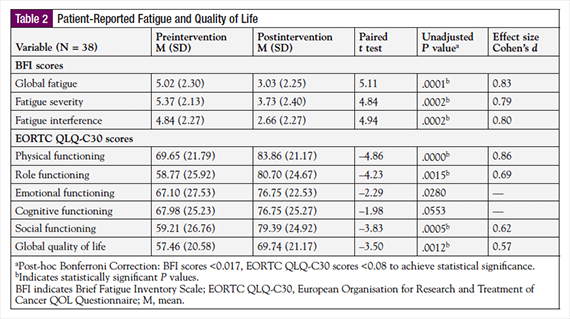
The effects of the PREP on CRF, functional status, and QOL outcome measures are shown in Table 2. There were significant reductions observed between pre- and post-PREP global CRF, severity of CRF, and CRF interference scores. Mean global CRF scores significantly decreased from pre-PREP (M = 5.02) to post-PREP (M = 3.03), with a mean paired difference of 1.99 (95% confidence interval [CI], 1.19, 2.77; t(37) = 5.11; P = .0000; d = 0.83). Similarly, severity of CRF scores demonstrated a significant decrease from pre-PREP (M = 5.37) to post-PREP (M = 3.73), with a paired mean difference of 1.64 (95% CI, 0.95, 2.32; t(37) = 4.84; P = .000; d = 0.79). The mean paired difference score for CRF interference was 2.18 (95% CI, 1.28, 3.07; t(37) = 4.94; P = .000; d = 0.80), which also constituted a significant reduction from pre-PREP (M = 4.84) to post-PREP (M = 2.66).
The PREP had a positive effect on several categories of functional status and overall QOL. All functional status scores increased after the PREP; however, only physical, role, and social functioning demonstrated a significant improvement. Physical functioning scores significantly increased from pre-PREP (M = 69.65) to post-PREP (M = 83.86), with a mean paired difference of –14.21 (95% CI, –20.13, –8.29; t(37) = –4.86; P = .0000; d = 0.86). The mean paired difference score for role functioning was –21.93 (95% CI, –32.13, –8.29; t(37) = –4.23; P = .0001; d = 0.67), which also suggested a significant increase from pre-PREP (M = 58.77) to post-PREP (M = 80.70). Social functioning scores also demonstrated a significant increase from pre-PREP (M = 59.21) to post-PREP (M = 79.38), with a mean paired difference of –30.84 (95% CI, –30.84, –9.51; t(37) = –3.83; P = .0005; d = 0.62). Cognitive and emotional functioning did not demonstrate a significant improvement from pre-PREP scores to post-PREP scores in this sample. Overall QOL (global health status/QOL) scores demonstrated a significant improvement from pre-PREP (M = 57.46) to post-PREP (M = 69.74), with a mean paired difference of –12.28 (95% CI, –19.39, –5.17, t(37) = –3.50; P = .0012; d = 0.57).
Although this study may not represent all early cancer survivors, our findings suggest that the PREP was effective in reducing patient-reported CRF, increasing physical, social, and role functioning, and improving QOL in this population of early cancer survivors. These benefits were not only found to be statistically significant, but also clinically meaningful with effect sizes ranging from d = 0.62 (moderate) to d = 0.83 (large).
Discussion
Of the 70 eligible cancer survivors who participated in this study, 38 (54%) completed the 60-day PREP. Despite researcher efforts, such as follow-up phone calls and use of a physician prescription to increase adherence to the exercise intervention in this study, 46% of the cancer survivors did not complete the 60-day PREP. Completion rates (54%) for this study are somewhat lower than previous studies that reported compliance rates of 61% with exercise interventions.14 However, statistical analysis demonstrated that there were no significant differences between cancer survivors who did and did not complete the PREP (in terms of age, sex, race, ethnicity, and cancer type), which suggested a reduced risk for response bias.
An important finding from this study was that the PREP reduced self-reported overall CRF, the severity of CRF, and the amount of interference CRF had on ADLs in early cancer survivors. This suggested that our study not only reduced the severity of fatigue, but also the inference that fatigue has on daily routines, meaningful social interactions, and the ability of survivors to maintain a sense of normalcy in their lives after cancer treatment. A study with a similar sample size and exercise intervention reported findings consistent with our results. This study consisted of an 8-week, supervised exercise program in deconditioned, early cancer survivors. Findings, in part, included significant reductions in fatigue from preintervention baseline to 3-month follow-up, and were reported as P = .04.27 Several studies that used exercise as an intervention also reported significant reductions in self-reported CRF.4,28,29
In keeping with the conceptual model for this study, defining CRF as a daily stressor often resulted in impairment in the variables and roles of self for cancer survivors. This included adverse effects on physiochemical functions of the body, mental processes and emotions, and social or cultural expectations and activities.20 An interesting finding from our study was the effect that the PREP had on improving physical, social, and role functioning, as well as overall QOL in early cancer survivors experiencing CRF. These findings are consistent with previous exercise interventional research on CRF that reported significant improvements in physical,4,28,29 role,29 and social functioning.4,29 There was no significant improvement in emotional and cognitive functioning in our study. Conversely, other researchers were able to demonstrate a significant benefit from their exercise interventions on emotional and/or cognitive functioning.4,28,29 In addition to reducing CRF, the primary goal of our study was to improve overall QOL for early cancer survivors. Improvements in QOL after completing an exercise intervention for CRF were also reported by other studies.4,29 For example, Litterini and Fieler reported a significant improvement in QOL (P = .000) following a twice-weekly, 10-week, physical therapist–supervised exercise program to reduce CRF.29
Strengths and Limitations
There were many strengths of this study; most notably, the PREP was implemented by CET-certified medical fitness specialists. The CET-certified medical fitness specialists were able to provide standardization and control to the exercise intervention, while individualizing the program based on each survivor’s health status. Another major strength of this study was the timing of the intervention during the early survivorship phase, when many patients still experience acute side effects and are more likely to be deconditioned from treatment. Although exercise interventional research in cancer populations is plentiful during the late survivorship phase, to the authors’ knowledge, only 1 other study has examined the effects of an exercise program on CRF during the early survivorship period. Thus, this study adds important insight to the body of literature about the benefits of exercise within 90 days of completing cancer treatment.
There were, however, some study limitations, including the use of a quasi-experimental designs, that did not allow for a randomized control group comparison. There were no differences in demographic and clinical characteristics among the cancer survivors who did and did not complete the PREP; however, the overall low completion rate (54%) is another limitation of this study. Therefore, future exercise-based interventional studies in cancer survivors should consider incorporating a feasibility analysis as an outcome measure of intervention success. Additional measures to increase adherence and completion of the intervention, such as small gifts (eg, gift cards), could also be considered. Other limitations of this study were the use of convenience sampling techniques to recruit early cancer survivors from 1 community cancer center, and a relatively small sample that consisted of an increased proportion of cancer survivors who were younger white women. Although these limitations are consistent with previous research studies, they reduce the generalizability of our findings nonetheless. The lack of sample diversity in this study suggests that recruitment efforts in future research on the effects of exercise on CRF should adopt strategies to enhance the recruitment of cancer survivors who are men from diverse racial and ethnic backgrounds.
Implications for Nursing Practice
This study confirmed the benefits of exercise in reducing CRF and improving QOL in early cancer survivors. Yet, many cancer survivors do not participate in recommended physical activity to assist in managing the effects of CRF, suggesting that more attention on this intervention is necessary.4 Communication gaps between patients and oncology providers were identified as a barrier to CRF management.30 Some patients accept CRF as an expected outcome of their illness, which may explain why CRF is underreported and undermanaged. Patients also fear that increasing CRF is a sign that their disease is getting worse and only report it when their ADLs and QOL are severely compromised.12 Effective patient–provider communication is essential for symptom management, and all patients should be assessed for the impact CRF has on their daily lives. Comprehensive assessment of CRF in survivors should include severity and frequency of CRF, identification of factors that influence CRF, the level of interference that CRF has on ADLs, recognition of barriers that may prevent adherence to CRF interventions, and identification of areas to direct patient and family guidance and education.
Conclusions
The findings from this study suggest that the PREP reduced CRF and improved QOL in early cancer survivors. These important findings add to the little evidence that has been published regarding the effectiveness of exercise to reduce CRF in early cancer survivors. All patients completing cancer treatment should be assessed for CRF and encouraged to enroll in a supervised exercise or rehabilitation program. More specifically, oncology nurses providing cancer survivorship care should assess patients for CRF when cancer treatment is completed. This may lead to early recognition and improved CRF outcomes. Oncology nurses are in a prime role to educate cancer survivors on the benefits of regular exercise to reduce CRF and improve QOL in the early posttreatment period.
Author Disclosure Statement
Ms Oertle and Ms Pirollo have no conflicts of interest to report. Dr Burrell is employed at Inspira Health Network and Villanova University.
References
- American Cancer Society. Cancer treatment & survivorship facts & figures 2014-2015. www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed August 25, 2015.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117-128.
- Brearley SG, Stamataki Z, Addington-Hall J, et al. The physical and practical problems experienced by cancer survivors: a rapid review and synthesis of the literature. Eur J Oncol Nurs. 2011;15:204-212.
- Rajotte EJ, Yi JC, Baker JS, et al. Community-based exercise program effectiveness and safety for cancer survivors. J Cancer Surviv. 2012;6:219-228.
- Poirier P. The impact of fatigue on role functioning during radiation therapy. Oncol Nurs Forum. 2011;38:457-465.
- Hoffman M, Ryan J, Figueroa-Mosely C, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(supp 1):4-10.
- Hsiech CC, Sprod LK, Hydock DS, et al. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008;35:909-915.
- Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91:822-828.
- National Comprehensive Cancer Network. NCCN guidelines: cancer-related fatigue. Version 2.2015. www.nccn.org/store/login/login.aspx ?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Accessed August 10, 2015.
- Yun YH, Lee KS, Kim YW, et al. Web-based tailored education program for disease-free cancer survivors with cancer-related fatigue: a randomized controlled trial. J Clin Oncol. 2012;30:1296-1303.
- Scott JA, Lasch KE, Barsevick AM, Piault-Louis E. Patients’ experiences with cancer-related fatigue: a review and synthesis of qualitative research. Oncol Nurs Forum. 2011;38:E191-E203.
- Given B. Cancer-related fatigue: a brief overview of current nursing perspective and experiences. Clin J Oncol Nurs. 2008;12(5 suppl):7-9.
- Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145.
- Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20;123-133.
- Mishra SI, Scherer RW, Snyder C, et al. The effectiveness of exercise interventions for improving health-related quality of life from diagnosis through active cancer treatment. Oncol Nurs Forum. 2015;42:E33-E53.
- Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
- Laino C. New guidelines for cancer patients from American College of Sports Medicine: exercising during & after treatment brings health benefits. Oncol Times. 2010;32:16-18.
- Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;1409-1426.
- Neuman B, Fawcett J. The Neuman Systems Model. 5th ed. Upper Saddle River, NJ: Pearson; 2011.
- American College of Surgeons. Commission on Cancer. www.facs.org/quality%20programs/cancer/coc. Accessed August 10, 2015.
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186-1196.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376.
- Berryman JW. Exercise is medicine: a historical perspective. Curr Sports Med Rep. 2010;9:195-201.
- American College of Sports Medicine. Certification. www.acsm.org/certification. Accessed August 10, 2015.
- Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139-150.
- Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7:551-562.
- Hanna LR, Avila PF, Meteer JD, et al. The effects of a comprehensive exercise program on physical function, fatigue, and mood in patients with various types of cancer. Oncol Nurs Forum. 2008;35:461-469.
- Litterini AJ, Fieler VK. The change in fatigue, strength, and quality of life following a physical therapist prescribed exercise program for cancer survivors. Rehabil Oncol. 2008;26:11-17.
- Piredda M, Laura R, Gualandi R. Cancer related fatigue and its management in patients. Healthcare professionals’ views: a literature review. Int Nurs Perspec. 2008;4:103-114.