Navigators can make a difference in negotiating the labyrinth of oncology clinical trials. Facilitating insurance coverage when a cancer patient chooses to enroll in a clinical trial is perhaps the trickiest part of the navigator’s role, said Wendy Portier, MSN, RN, CCM, CPHQ, CHC, CHRC.
In her presentation at the East Coast Regional Meeting of the Academy of Oncology Nurse & Patient Navigators, Ms Portier discussed regulations related to insurance coverage of clinical trials and how to remove barriers to such coverage.
Low Rate of Participation
At the micro level, clinical trials improve access to cutting-edge therapies. At the macro level, they advance medicine with evidence and benefit humanity. But fewer than 5% of cancer patients enroll in clinical trials, although one-third of American adults indicate willingness to participate.
In contrast, 60% of children younger than 15 years participate in clinical trials. “This is one main reason that survival rates for childhood cancer have improved so dramatically in the last few decades,” said Ms Portier, Managing Director at Portier & Associates, LLC, in New Orleans, LA. “One of the biggest barriers to completing clinical trials is that we have low participation rates.”
Low participation in clinical trials means that research takes longer, the generalizability of the results may be limited, the trials may be canceled prematurely, the development of new drugs is delayed, the cost of conducting trials increases, and the time required to get effective treatments into widespread practice is increased.
The most common reasons patients cite for declining participation in a clinical trial is fear of adverse effects (50%), discomfort with random assignment (44%), and concerns about added costs (28%). Cost was cited by 12% as the most important factor in their decision not to participate.
“Financial discussions need to happen with people that are really versed in that world,” said Ms Portier. Navigators must also be familiar with informed consent documents, which can reach 30 pages.
Locating a Trial
Navigators can assist with locating a clinical trial for their patients. ClinicalTrials.gov is a registry and results database that also lists trials for cancer. It contains trials supported by the National Cancer Institute (NCI) as well as trials sponsored by pharmaceutical, device, and biotechnology companies. On the NCI website, a search can be performed for clinical trials by geographic location.
A 10-step guide written to help consumers/patients find a clinical trial can be found at ericott.pbworks.com/f/treatment-trial-guide-pdf.pdf. Answers to questions related to informed consent and cost are covered in Step 8. Patients usually want to know about their responsibility for costs incurred before making a decision on entering into a clinical trial, said Ms Portier.
Navigating Insurance Coverage
Navigating insurance coverage for a clinical trial is a challenge, she said. Medicare has a Clinical Trial Policy in which it may cover the routine costs of qualifying clinical trials if they are: 1) not paid for by the sponsor, 2) not promised free in the informed consent form, and 3) are costs typically covered by Medicare absent a clinical trial. In this instance, routine costs include conventional care; detection, prevention, and treatment of complications; and the administration of an investigational agent.
Coverage of a clinical trial under Medicare is determined by a coverage analysis. If national or local coverage decisions under Medicare support billing of a test or procedure, it should be covered within the context of a clinical trial. Additionally, justification for coverage may also be found in National Comprehensive Cancer Network guidelines.
In 2014, the Affordable Care Act (ACA) mandated clinical trial insurance coverage. The mandate states that health plans or insurers must provide coverage to any qualified individual for routine patient costs of items or services furnished in connection with participation in an approved clinical trial for cancer or other life-threatening conditions.
An approved clinical trial is defined as a phase 1, 2, 3, or 4 clinical trial that is federally funded or approved; a trial or investigation that is conducted under an investigational new drug (IND) application reviewed by the FDA; or a trial that is IND exempt.
Under the ACA, coverage requirements extend to most third-party payers of health benefits or insurance claims, health insurance exchanges plans and employer-sponsored plans, and Employee Retirement Income Security Act plans, unless they have grandfathered status.
For approved clinical trials, under the ACA, health plans can’t deny a person the right to take part in a clinical trial; limit, deny, or impose additional conditions on routine patient costs for items or services connected with the clinical trial; or discriminate against a person on the basis of their participation in a clinical trial (drop or limit coverage if an individual chooses to participate in a clinical trial).
“[Insurers] don’t have to pay for the actual investigational agent, the device, or the service that's being studied,” Ms Portier said. “They don’t have to pay for data collection or data analysis. They don’t have to pay for anything that’s not clearly aligned with standard of care. They don't have to pay for anything that’s not under the benefit plan.”
In addition to benefits verification, 2 other key clinical trial insurance coverage steps are network verification (the ACA does not guarantee individuals access to healthcare providers who are not participating in a health plan or insurer’s network) and preauthorization, which must occur per the patient’s individual plan requirements (Table).
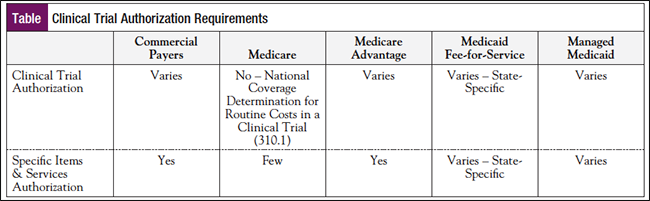
“A lot of commercial payers are now requiring authorization on the clinical trial level, and individual authorization for all the items and services throughout that clinical trial,” she said. “Really understand what that payer is requiring for authorization and submit a packet.”
Best practices for authorization request include creation and submission of an initial request packet to the insurer as required and financial counseling for potential clinical trial participants. The packet should include a protocol synopsis, a signed institutional review board (IRB)-approved informed consent form, a current IRB approval letter, a coverage analysis, applicable references including Medicare local/national coverage decisions, a clinical trial request form and cover letter, and a primary contact at the clinical trial site.
If coverage is denied, the ACA allows patients and providers to file an appeal. First, an appeal must be submitted to the health plan that denied the coverage for the requested treatment. An external appeal can be filed if internal appeal options are exhausted. With an external appeal, an independent review organization acts as a third-party review source.




