Addressing barriers to clinical trial participation is critical to accelerate progress toward more effective (and less toxic) cancer treatments and provide patients with access to novel treatment approaches.1 Receiving treatment in a clinical trial is considered by many to be high-quality cancer care2; the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology state that “the best management for any cancer patient is in a clinical trial.”3
Unfortunately, access to and participation in these trials is inequitable. While about 20% of all cancer patients are eligible to participate in cancer treatment trials, only about 8% participate.4,5 It has been estimated that only 15% of those participating are from racial and ethnic minority groups.6,7 These low participation rates may well perpetuate disparities in treatment outcomes and continue to lead to limited generalizability for newly discovered cancer treatments,5,6,8 further perpetuating health inequities.9
The literature has documented numerous barriers to cancer clinical trial participation, particularly for patients from ethnic and racial minority groups.5,10-19 These include: (1) institutional and provider-related barriers, such as the availability of local trials, awareness of trials outside local cancer centers, staff/infrastructure capacity and capability (including time and resources to identify potential trials), quality and variability of provider communication, ineffective patient identification and enrollment practices, and fear of losing the patient to another organization or provider; (2) barriers related to trial design, such as restrictive inclusion or exclusion criteria, lack of patient-centeredness, location of trial site, and limited funding to support participation; and (3) patient-level barriers, including awareness, self-efficacy, fear and mistrust, a preference not to lose control of treatment decision-making, costs (both real and perceived), and logistical concerns, such as travel, time off work, or family responsibilities.
While many of these barriers are systemic and multilevel, nurse navigators—particularly those who work in institutions participating in treatment trials—have a unique and important role in addressing some of these barriers, especially those that disproportionately affect patients from minority groups.
Several studies have shown that patient navigation in clinical trials—with a focus on increasing awareness, knowledge, facilitating access to community resources, and improving communication between the patient and the treatment team20—can make a difference in increasing trial participation. In its various forms, navigation has been shown to increase patient awareness and knowledge about clinical trials21,22 and participation in trials, with several studies showing increased trial accrual and retention rates among patients from minority groups.20,23-28
Roles of the Nurse Navigator in Cancer Treatment Trials
Below we outline 2 key roles for nurse navigators to consider incorporating into their work.
Role 1: Educate All Patients About Cancer Clinical Trials and Encourage Inquiry
Although there are documented system-, patient-, and provider-level barriers to clinical trial participation, a critical challenge is that trials are not consistently offered to all potentially eligible patients.
When conducting cancer treatment trials, consistent and systematic screening for eligibility is critical; all patients found to be eligible should be asked about their interest in participating.13 Although this is best practice, it is not reality. Several studies have shown that even when found eligible, many patients are never approached about the option.17,29-31 Moreover, bias and stereotypes lead to the perception that patients from minority groups are not interested or are simply poor study candidates.7 Numerous studies have shown that when asked, most patients with cancer from ethnic and racial minority groups are as willing to participate in cancer clinical trials as Whites.30,32-36
Nurse navigators have an important role in educating and empowering patients to ask about clinical trials. For example, as a part of their assessment, they can:
- provide education about available treatment options, including the possibility of receiving treatment through a clinical trial
- clarify differences between standard of care and treatment within a trial
- “normalize” clinical trials as a high-quality option for cancer care (eg, “last year, 250 patients at our site decided to get their treatment in a clinical trial”)
- acknowledge distrust and provide reassurance
- provide education about the clinical trial process (eg, how informed consent works, why people may be assigned to treatment groups)
- address fears, concerns, and myths about clinical trials
- and perhaps most important, encourage inquiry about the possibility of receiving treatment through a clinical trial
Nurse navigators can also serve as “translators” to debrief discussions with the treatment team. With patient permission, they can sit in on treatment discussions and afterward help clarify any information that may have been confusing.
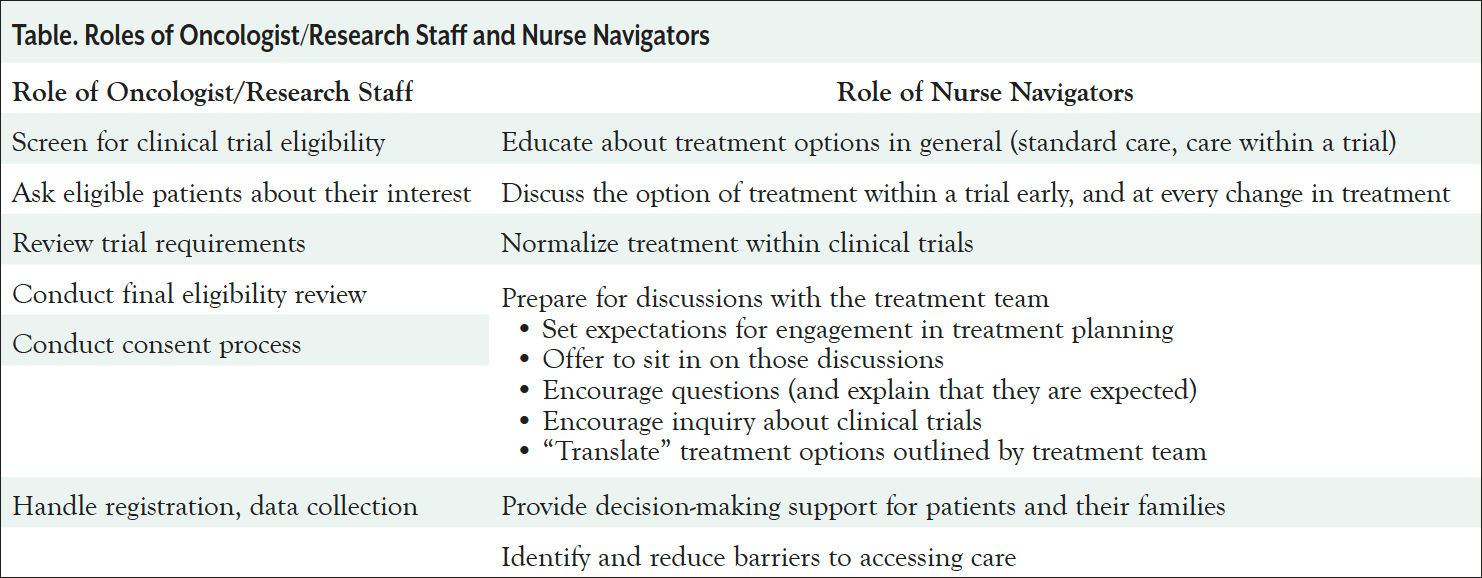
It is important to note that the nurse navigator complements the oncologist and research staff roles. While oncologists and research staff are responsible for discussing specific clinical trials for which patients have been found eligible and conducting the consent process, nurse navigators can educate about clinical trials in an unbiased way. Some of the key differences in roles are listed in the Table.
Role 2: Proactively Address Logistical Challenges to Enrollment and/or Retention for Patients Interested in Trial Participation
An important role of the nurse navigator is identifying and addressing barriers to the timely receipt of care to avoid unnecessary delays in treatment; the same holds true when accessing treatment in a clinical trial.
As noted in a study by Freund and colleagues,27 addressing social needs is both complex and challenging. It requires the navigator to develop a plan of action, such as helping patients complete applications and reaching out to external organizations for financial assistance and resources. It also requires following up with the patient and, depending on the lack of resolution, providing support and resources for an alternative treatment plan. For clinical trials, addressing logistical challenges must begin at the time of the treatment discussion and before the consent process and continue during the consent and enrollment process, retention throughout treatment on the trial, and until the start of follow-up care after the conclusion of the trial. These challenges include:
- Identifying logistical barriers or social needs (eg, screening for social determinants of health,27 educational assessment, identifying psychosocial and financial supports, child/dependent care needs)
- Addressing barriers (eg, transportation, identifying financial and social support resources for patient and caregiver, and child care support services) so a patient can access treatment in a timely manner
- Facilitating enrollment in health insurance/Medicaid/Medicare and navigating the insurance appeals process
Conclusion
Patient navigation—in all its many forms—has been shown to improve care for patients with cancer, particularly patients from ethnic and racial minority groups, patients with linguistic challenges, patients from low-income neighborhoods, and patients who are uninsured or underinsured.26,37-41 Navigation programs have been shown to reduce delays in cancer care, increase delivery of cancer care that is guideline concordant, and reduce hospitalization or emergency department care during cancer treatment.37,38,41-43 Patient navigation in clinical trials is an important component of cancer treatment navigation, showing promise in increasing patient awareness and knowledge21,22 as well as increasing trial participation.20,22-25,44,45 Empowering more nurse navigators to focus on cancer treatment trials as a part of treatment navigation may well have an impact on reducing cancer and cancer care disparities.
References
- Stensland KD, McBride RB, Latif A, et al. Adult cancer clinical trials that fail to complete: an epidemic? J Natl Cancer Inst. 2014;106:dju229.
- Oyer RA, Hurley P, Boehmer L, et al. Increasing racial and ethnic diversity in cancer clinical trials: an American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. J Clin Oncol. 2022;40:2163-2171.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines. https://jnccn.org/view/journals/jnccn/12/11/article-p1526.xml.
- Unger JM, Vaidya R, Hershman DL, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111:245-255.
- American Cancer Society Cancer Action Network. Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer: A Landscape Report. www.fightcancer.org/sites/default/files/National%20Documents/Clini cal-Trials-Landscape-Report.pdf. 2018. Accessed March 31, 2023.
- Duma N, Aguilera JV, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14:e1-e10.
- Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126:1958-1968.
- Regnante JM, Richie N, Fashoyin-Aje L, et al. Operational strategies in US cancer centers of excellence that support the successful accrual of racial and ethnic minorities in clinical trials. Contemp Clin Trials Commun. 2020;17:100532.
- Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71:78-92.
- Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105-114.
- Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;36:185-198.
- Heller C, Balls-Berry JE, Nery JD, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials. 2014;39:169-182.
- Oyer RA, Hurley P, Boehmer L, et al. Increasing racial and ethnic diversity in cancer clinical trials: an American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. J Clin Oncol. 2022;40:2163-2171.
- Hamel LM, Penner LA, Albrecht TL, et al. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23:327-337.
- Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228-242.
- Ford JG, Howerton MW, Bolen S, et al. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evid Rep Technol Assess (Summ). 2005;(122):1-11.
- Guarino MJ, Masters GA, Schneider CJ, et al. Barriers exist to patient participation in clinical trials. J Clin Oncol. 2005;23(suppl). Abstract 6015.
- Barry D, Steinberg JR, Towner M, et al. Enrollment of racial and ethnic minoritized groups in gynecologic oncology clinical trials: a review of the scope of the problem, contributing factors, and strategies to improve inclusion. Clin Obstet Gynecol. 2023;66:22-35.
- Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Cancer Netw. 2007;5:753-762.
- Ghebre RG, Jones LA, Wenzel JA, et al. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer. 2014;120(suppl 7):1122-1130.
- Moffitt K, Brogan F, Brown C, et al. Statewide cancer clinical trial navigation service. J Oncol Pract. 2010;6:127-132.
- Cartmell KB, Bonilha HS, Matson T, et al. Patient participation in cancer clinical trials: a pilot test of lay navigation. Contemp Clin Trials Commun. 2016;3:86-93.
- Fouad MN, Acemgil A, Bae S, et al. Patient navigation as a model to increase participation of African Americans in cancer clinical trials. J Oncol Pract. 2016;12:556-563.
- Wujcik D, Wolff SN. Recruitment of African Americans to national oncology clinical trials through a clinical trial shared resource. J Health Care Poor Underserved. 2010;21(suppl):38-50.
- Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials. 2009;6:610-617.
- Ramirez A, Perez-Stable E, Penedo F, et al. Reducing time-to-treatment in underserved Latinas with breast cancer: the Six Cities Study. Cancer. 2014;120:752-760.
- Freund KM, Haas JS, Lemon SC, et al. Standardized activities for lay patient navigators in breast cancer care: recommendations from a citywide implementation study. Cancer. 2019;125:4532-4540.
- Barrett N, Boehmer L, Schrag J, et al. Assessing feasibility and utility of an implicit bias training program for addressing disparities in cancer clinical trial participation. J Clin Oncol. 2022;40(suppl). Abstract e18599.
- Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106:426-433.
- St Germain DC, McCaskill-Stevens W. Use of a clinical trial screening tool to enhance patient accrual. Cancer. 2021;127:1630-1637.
- Albrecht TL, Eggly SS, Gleason MEJ, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666-2673.
- Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. Gerontologist. 2003;43:62-72.
- Langford AT, Resnicow K, Dimond EP, et al. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer. 2014;120:877-884.
- Unger JM, Hershman DL, Till C, et al. “When Offered to Participate”: a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J Natl Cancer Inst. 2021;113:244-257.
- Brandon DT, Isaac LA, LaVeist TA. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc. 2005;97:951-956.
- Katz RV, Kegeles SS, Kressin NR, et al. Awareness of the Tuskegee Syphilis Study and the US presidential apology and their influence on minority participation in biomedical research. Am J Public Health. 2008;98:1137-1142.
- Percac-Lima S, Ashburner JM, Zai AH, et al. Patient navigation for comprehensive cancer screening in high-risk patients using a population-based health information technology system: a randomized clinical trial. JAMA Intern Med. 2016;176:930-937.
- Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014;32:2758-2764.
- Ko NY, Snyder FR, Raich PC, et al. Racial and ethnic differences in patient navigation: results from the Patient Navigation Research Program. Cancer. 2016;122:2715-2722.
- Rodday AM, Parsons SK, Snyder F, et al. Impact of patient navigation in eliminating economic disparities in cancer care. Cancer. 2015;121:4025-4034.
- Rocque GB, Williams CP, Jones MI, et al. Healthcare utilization, Medicare spending, and sources of patient distress identified during implementation of a lay navigation program for older patients with breast cancer. Breast Cancer Res Treat. 2018;167:215-223.
- Percac-Lima S, Ashburner JM, Zai AH, et al. Patient navigation for comprehensive cancer screening in high-risk patients using a population-based health information technology system: a randomized clinical trial. JAMA Intern Med. 2016;176:930-937.
- Freund KM, Battaglia TA, Calhoun E, et al. Impact of patient navigation on timely cancer care: the Patient Navigation Research Program. J Natl Cancer Inst. 2014;106:dju115.
- Sae-Hau M, Disare K, Michaels M, et al. Overcoming barriers to clinical trial participation: outcomes of a national clinical trial matching and navigation service for patients with a blood cancer. JCO Oncol Pract. 2021;17:e1866-e1878.
- Holmes DR, Major J, Lyonga DE, et al. Increasing minority patient participation in cancer clinical trials using oncology nurse navigation. Am J Surg. 2012;203:415-422.
Resources
Free resources that can help educate your patients and, in some cases, assist them in finding clinical trials:
For All Cancers
- Cancer Support Community cancer support helpline (ask for clinical trials navigator). Call 888-793-9355
- EmergingMed Clinical Trial Navigator Service includes an online search tool to look for clinical trials. Patients can also get help from a clinical trial navigator through their website (app.emergingmed.com/emed/home)
- National Cancer Institute provides an online search tool for cancer clinical trials at cancer.gov/about-cancer/treatment/clinical-trials. Patients can get help with the search by calling 800-422-6237
- Cancer Support Community Peer Clinical Trials Support Program (for Black or African American cancer patients) www.cancersupportcommunity.org/peer-clinical-trials-support-program
For Blood Cancers
- The Leukemia & Lymphoma Society Clinical Trial Support Center. www.lls.org/support-resources/clinical-trial-support-center-ctsc. Call 800-955-4572 or This email address is being protected from spambots. You need JavaScript enabled to view it.





