Background: Nurse navigators have been described as the “anchor” of the multidisciplinary team. Molecular testing is an increasingly important component of non–small cell lung cancer (NSCLC). To facilitate appropriate and timely molecular testing, nurse navigators must have a robust understanding of molecular testing and associated processes.
Objectives: To describe types of molecular testing and discuss considerations for their application in NSCLC to support the role of the nurse navigator in facilitating efficient molecular testing.
Methods: A review and synthesis of available literature were conducted. Monthly collaborative discussions occurred between the authors from March to September 2023. A case study is presented to highlight the value of the nurse navigator in appropriate and timely molecular testing.
Findings: Based on this work, a list of recommendations was compiled to support nurse navigators who wish to play an active role in facilitating appropriate and timely molecular testing for NSCLC patients.
In the United States, lung cancer is the leading cause of cancer mortality.1 While most lung cancers are attributable to combustible tobacco, an increasing number are seen in people who have never been exposed to combustible tobacco.2 Non—small cell lung cancer (NSCLC), including adenocarcinoma, squamous cell carcinoma, and several additional subvariants, represents over 75% of cases.3 Unfortunately, up to 75% of NSCLC cases are metastatic at diagnosis, and just 6% of these patients survive 5 years beyond diagnosis.1,4
Diagnosis is confirmed by bronchoscopic, percutaneous, or surgical biopsy of the site of highest suspected disease identified on CT or PET imaging.5 Once pathologic diagnosis is confirmed, staging is completed with PET imaging, if not completed prior to biopsy, and brain MRI.6 NSCLC stage and identification of targetable variants through molecular testing guides treatment decisions.6
Molecular Testing
Molecular testing is the process of identifying targetable variants in tumor cells, and it has recently seen dramatic changes. It is a complex, evolving, and important part of NSCLC care that requires a coordinated multidisciplinary team and programmatic approach. Nurse navigators often coordinate care, and molecular testing in NSCLC is no exception.
Before the evolution of molecular testing in NSCLC, the benchmark time from pathologic diagnosis to treatment was 42 days.7 Today, this benchmark is increasingly narrow, with benchmarks now under 30 days.8 However, obtaining molecular testing results can take over half of the current benchmark time.9 Nurse navigators play a critical role in coordinating care for NSCLC, which includes ensuring molecular testing is accurately and efficiently completed. Prolonging the diagnosis to treatment timeline negatively impacts treatment outcomes.
Targetable Variants
Targetable variants are genome alterations or driver variants present in cancer cells for which a targeted therapy is available.10 Targetable or actionable mutations for NSCLC include PD-L1, EGFR, KRAS, ALK, ROS1, BRAF, NTRK, MET, RET, and ERBB2.6,11,12
Guidelines recommend identifying targetable variants because targeted therapies improve survival.5,6,10 More than 73% of NSCLC cases with an EGFR mutation treated with an EGFR tyrosine kinase inhibitor experienced prolonged survival.13 Advanced NSCLC cases with PD-L1 expression over 50% treated with pembrolizumab plus platinum-based chemotherapy experience greater survival and reduced adverse effects than with chemotherapy alone.5
Types of Molecular Testing
Molecular testing is recommended at diagnosis for advanced NSCLC.5 Nurse navigators should understand the types of tests available and facilitate their appropriate use in practice. Standardized language should be used to ensure the appropriate test is ordered, the results are interpreted correctly, and the treatment plan is understood.9,14 Standardized language improves communication and decreases the risk of error.14
Germline Testing
Germline testing evaluates genetic changes in normal cells. Also called hereditary variants, these changes are present in all cells and are used to identify variants that increase the risk for cancer or predict pharmacogenomic response to therapy.15 Germline testing plays only a small role in NSCLC. However uncommon, NSCLC is increasing in patients who have never used combustible tobacco, and germline testing may play a larger role in the future.2
Somatic Testing
Unlike germline variants, somatic variants are not hereditary and are present only in tumor cells.15 Somatic variants are acquired, not inherited. More than 80% of lung cancer cases are caused by combustible tobacco use, the most common cause of somatic variants in NSCLC.2 Somatic testing in NSCLC is conducted on tumor cells collected during bronchoscopy, percutaneous biopsy, or diagnostic surgical resection or by identifying circulating tumor DNA (ctDNA) in the blood.16
Molecular Testing on Tumor Tissue
Molecular testing on tumor tissue is the gold standard and should be performed on the tumor itself or a metastatic site.5,6,10,17,18 Limitations of testing tumor tissue include risks associated with biopsy and quantity not sufficient (QNS) for molecular testing after an invasive procedure.18
Molecular Testing by Liquid Biopsy
Liquid biopsy is molecular testing that identifies ctDNA in the blood. ctDNA is DNA fragments that moved from tumor tissue into the blood during apoptosis or necrosis, which are best identified in the context of advanced NSCLC, although it is under study for use in earlier stages.16,18 Liquid biopsy may be appropriate in conjunction with molecular testing on tumor tissue, when the patient cannot undergo biopsy, or in cases of QNS for molecular testing.6,17,19 It also has potential for monitoring response to therapy.20
Molecular Testing Process
Molecular testing is complex. Challenges include determining the appropriate test, informing the patient about the test, obtaining sufficient tissue to perform the test, identifying which laboratory will perform the test, determining which provider will order the test, identifying financial resources to support the patient, coordinating multiple specialties, and communicating with reference laboratories.9
In a retrospective review of 479 patients tested for EGFR and ALK, the median time from diagnosis until the molecular testing result was received by the care team was 23 days, exceeding the recommended 14 days.9 It is important to recognize the molecular testing process length relative to the benchmark time from diagnosis to treatment.6-8 Nurse navigators should understand that claims about time to results often capture time from reference laboratory receipt of tumor cells to results reported within their documentation. These claims infrequently account for the time required to prepare tissue for processing, ship tissue to the laboratory, and communicate the results to the care team. The process steps from cell collection to results available to treating oncologist when using a reference laboratory are presented in Figure 1.
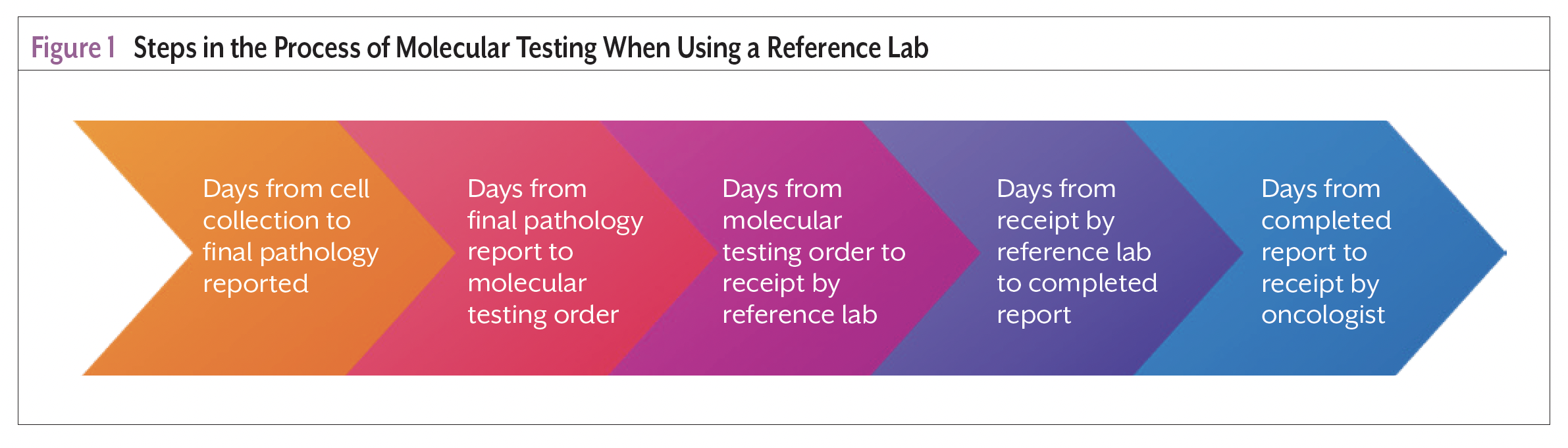
Considerations for Molecular Testing
Molecular testing requires a coordinated multidisciplinary approach. There are a multitude of factors to consider when planning quality NSCLC care. Considerations for molecular testing are summarized here.
Determining Appropriate Test
The appropriate test identifies or rules out variants needed to select the best treatment for a particular NSCLC stage or tumor size. EGFR variant testing is recommended in IB-IIIA NSCLC cases because treatment with an EGFR tyrosine kinase inhibitor improves survival.10,13 In addition to EGFR, ALK and PD-L1 testing is recommended in IB-IIIA cases with tumors measuring at least 4 cm to identify potential candidates for neoadjuvant therapy, which results in an improved response.21 Broad molecular testing is recommended on all advanced or metastatic NSCLC cases.6,12,22 Broad molecular testing includes EGFR, ALK, KRAS, ROS1, BRAF, NTRK1/2/3, METexon14 skipping, RET, and ERBB2 (HER2), which are commonly identified using next-generation sequencing (NGS).6,12,22
Gaps in insurance coverage have not been found to be a significant barrier because of reference laboratory policies that cap out-of-pocket expenses and offer financial assistance for patients unable to afford testing.
NGS identifies multiple genetic variants simultaneously.10,23 It provides more comprehensive information using fewer cells than tests that evaluate for single genetic variants, making it more cost-effective.2,5,10,19,24 NGS also identifies less common genetic variants and measures their levels of expression.10,19 PD-L1 is assessed by immunohistochemistry, a different technology that requires an additional 50 to 100 cells and is often reported alongside NGS testing on tumor cells.5,6,10,19
Using a Reference Laboratory
A reference laboratory is a laboratory that offers specialized testing and new diagnostic tests.25 Although the availability of molecular testing is problematic for most of the world, technology improvements and the growth of these laboratories have improved accessibility, minimizing differences between urban and rural healthcare settings.19,26
Some institutions identify genetic variants utilizing a test available within the institution, which may decrease turnaround time and improve access to a wider variety of sample specimens that are not commonly sent to external labs.10,27,28 Challenges affecting both institutional and reference laboratories include the sample processing; sample quality and quantity; management of data; and staff experience, capabilities, and education.29 Optimizing laboratory processes contributes to optimal outcomes.29
CLIA-Accredited Laboratory
In the United States, any laboratory used for molecular testing should be, at a minimum, Clinical Laboratory Improvement Amendments (CLIA) accredited.6 The FDA, Centers for Medicare & Medicaid Services (CMS), and the Centers for Disease Control and Prevention are responsible for CLIA and regulate clinical laboratories that accept human samples for diagnostic testing to ensure quality standards are met.30
FDA-Approved Tests
FDA-approved molecular tests include FoundationOne CDx, PGDx elio tissue complete, Oncomine Dx Target Test for testing tissue and Guardant 360, Agilient Resolution ctDx FIRST, and FoundationOne Liquid CDx for testing blood.2,5,19,31 Although FDA approval is not required for coverage by all insurers, it is required under the Medicare NGS coverage policy.32 Furthermore, FDA approval is described in the indications of many drug labels of targeted therapies for NSCLC.
Understanding Financial Barriers
CMS determined NGS as a reasonable and necessary diagnostic laboratory test in 2018. The test is covered when performed in a CLIA-certified laboratory; when ordered by a treating physician; and when the cancer is recurrent, relapsed, refractory, metastatic, or advanced stage III or IV that has not been previously tested with the same NGS test for the same cancer genetic content, and when the patient will continue cancer treatment.32
Although the utility of these tests has been recognized by and incorporated into clinical guidelines,6 payer coverage policies have historically been more variable.33 It is important for ordering physicians to have a relationship and understanding of the individual reference laboratory’s billing and financial assistance programs. Many reference laboratories have dedicated service teams to work with patients’ insurance companies to include claim submission and follow-up if a denial is received. It is beneficial for patients to complete financial assistance forms at the time of testing to facilitate this process.34 Gaps in insurance coverage have not been found to be a significant barrier because of reference laboratory policies that cap out-of-pocket expenses and offer financial assistance for patients unable to afford testing.33 Another important financial consideration is the Laboratory Date of Service Policy.35 This rule requires molecular tests to be ordered at least 14 days after a hospital discharge to avoid cost associated with bundling these tests with inpatient costs.
Ordering the Test
Informed consent is an ethical and legal obligation of healthcare providers.36 Some molecular testing orders require the ordering provider to acknowledge that informed consent was completed.28 Informed consent is complex in regard to molecular testing because the risks, benefits, and alternatives continue to evolve.36 Opportunities to obtain informed consent include when consent is obtained for a biopsy procedure, when final pathology is communicated to the patient, or when a molecular test is ordered. Practice resources to facilitate molecular testing discussions are available in the Table.
Payers, reference laboratory policies, and cancer care system practices impact which provider orders molecular testing. CMS requires the test to be ordered by the treating provider or the pathologist via standing order.32 Although medical oncologists often drive molecular testing, patients may wait weeks for initial consultation, and relying on medical oncologists to order testing can impact guideline adherence and prolong time to complete molecular testing.
Molecular testing should be initiated at time of diagnosis,5 and CMS allows for pathologists to initiate molecular testing based on standing orders.32 Pathologists are an increasingly important player in the decision-making process and must be knowledgeable about molecular testing to support timely and efficient molecular testing.37 Nurse navigators play an important role in ensuring informed consent, connecting patients with financial resources to overcome financial barriers, and advocating for standardized processes that improve appropriate and timely molecular testing.
Mitigating QNS
While nurse navigators do not directly influence QNS results, understanding the impact of this issue can help nurse navigators inform patients, facilitate timely communication for a plan of care when QNS does occur, and advocate for evidence-based practice. The most common reason molecular testing is not conducted is QNS.9 Minimally invasive techniques are used to diagnose most advanced lung cancer cases.5 Although these techniques improve outcomes, they may not collect enough cells for molecular testing.6 Ordering a second molecular test further prolongs the time from diagnosis to treatment.2
Payers, reference laboratory policies, and cancer care system practices impact which provider orders molecular testing. CMS requires the test to be ordered by the treating provider or the pathologist via standing order.
Molecular testing requires 100 to 400 tumor cells and a sample of at least 20% tumor cells.5 QNS for molecular testing can occur in up to 38% of bronchoscopies.5 QNS can occur with the quantitatively or qualitatively insufficient collection of tumor cells during biopsy or with processing practices by laboratories that utilize cells inefficiently. Reattempting biopsy to collect more tumor cells is not ideal due to the risks and financial considerations associated with biopsy procedures.18
Reflex to liquid is a model used by some health systems to mitigate QNS. This model uses QNS as automatic or reflexive order for liquid biopsy and can reduce time to treatment.38 Guidelines encourage biopsy techniques that maximize tissue for molecular testing, including protocols for small biopsy and collaboration between proceduralist and pathologist during the procedure,5,6,38 and utilizing liquid biopsy if tissue is not available.6 As biopsies are frequently performed outpatient, this model requires coordination and an understanding of Medicare coverage, which may limit the use of 2 NGS tests that seek to identify the same genetic variants.32 Reflex to liquid requires blood sample collection during tumor cell collection and preservation until needed, or at the time the liquid biopsy is ordered.
Working With a Reference Laboratory
Laboratory Representatives
Laboratory representatives can be extremely valuable resources for acquiring current information regarding evolving technologies. Successfully navigating reference laboratory relationships is paramount to ensure access to quality molecular testing and promoting best practices. The potential for bias exists in these relationships. Nurse navigators must understand that laboratory representatives are promoting a business.
Communicating information between a reference laboratory and a health system requires adherence to the Health Insurance Portability and Accountability Act’s Privacy Rule. This process is often cumbersome and inefficient.
Contracting
A formal agreement with a reference laboratory can improve molecular testing processes. Familiarity improves ease of use. However, this should not take priority over molecular testing quality, and contracts should outline quality measures and plans of action if they are not met. Measures such as an acceptable number of QNS results returned from the reference laboratory should be clearly defined in the contract. If not met, the cancer care system can reserve the right to pilot a test offered by a different laboratory if the reference lab is not meeting their needs.
Nurse navigators should critically evaluate ethics associated with contracting with a reference laboratory and an order that dictates the use of a particular reference laboratory. The ordering provider may rationalize that a particular company produces desired results more frequently, but these claims require data support through ongoing evaluation.
HIPAA
Communicating information between a reference laboratory and a health system requires adherence to the Health Insurance Portability and Accountability Act’s (HIPAA) Privacy Rule.39 This process is often cumbersome and inefficient. Results are often faxed or manually retrieved through the reference laboratory’s password-protected portal, both of which cause delays in receiving results and interrupt continuity of care when the ordering provider is not the treating provider. Variations in electronic medical records limit standardizing where results appear for the treating provider, and the process of getting results into the electronic medical record remains a challenge.40
Organizational processes for integrating new technology and collaboration between nurses, clinical teams, information services, and reference laboratories should be utilized to standardize processes, including ordering, viewing testing status, and communicating results into the electronic health record in a way that is accessible to all providers.
Case Study
A 59-year-old male was referred to a pulmonary nodule clinic with an 8.2×5.9×6.5-cm lung mass. A thoracic nurse navigator reviewed the case and recognized that if a biopsy were positive for NSCLC, the cancer stage would be at least IIIA. The pulmonologist and thoracic nurse navigator reviewed the case together. When the pulmonologist recommended a consult visit followed by bronchoscopy, the nurse navigator asked if they should anticipate PET scan, brain MRI, oncology referrals, and molecular testing per the institution’s NSCLC protocol. The pulmonologist agreed.
The thoracic nurse navigator connected the patient, who was underinsured at referral, with financial resources to avoid financial toxicity. The thoracic nurse navigator met the patient at the pulmonology consult visit and coordinated the bronchoscopy. The thoracic nurse navigator communicated with the pulmonologist when the final pathology report was available and coordinated the multidisciplinary conference where the patient case was presented.
The final pathology report showed NSCLC adenocarcinoma in samples of the mass and 1 lymph node. Molecular testing was ordered the same day the final pathology results were released. The PET scan and brain MRI were reviewed by the oncologist, who determined the patient had stage IIIC NSCLC adenocarcinoma and recommended a combination of nivolumab, ipilimumab, pemetrexed, and carboplatin, pending molecular testing results.
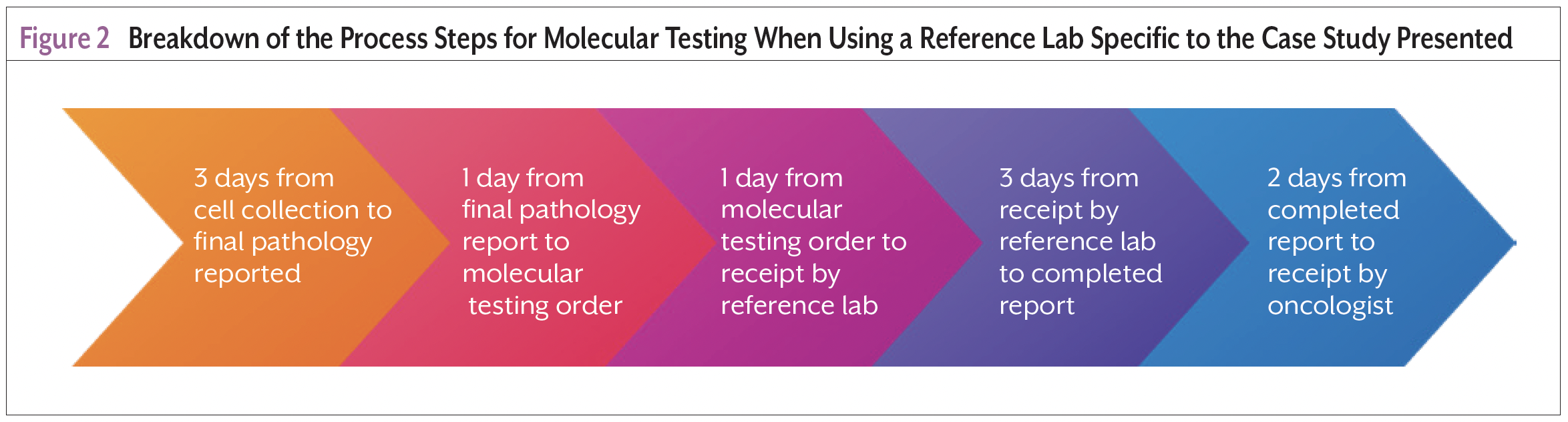
Ten days from the date of the bronchoscopy (Figure 2), the molecular testing results showed 90% PD-L1 expression. The nurse navigator retrieved the results from the reference laboratory’s documentation system and communicated with the medical oncologist. The patient received his first cycle of combination chemotherapy and immunotherapy 28 days after diagnosis.
Recommendations, Discussion, and Nurse Navigator Implications
An ideal molecular test identifies targetable variants with 100% accuracy, communicates results seamlessly without delaying treatment, creates no financial burden for the patient, and presents no safety risk. Unfortunately, no molecular tests are ideal, and integrating associated processes requires a robust understanding of influencing factors and coordination of efforts. Molecular testing is necessary because identifying targetable variants impact posttreatment disease progression and represent missed opportunities for targeted therapies that prolong survival.5,9,10
Utilizing nurse navigators facilitates care coordination.8 A multidisciplinary team is a group of experts from different specialties who work together to determine best possible plans of care, reduce time from diagnosis to treatment, and their implementation is associated with improved survival.41,42 Nurse navigators are an important part of this team, functioning as the “anchor” because they improve communication and collaboration, coordinate care, and overcome barriers.42
Nurse navigators must understand molecular testing terminology, which variants are identified with molecular tests used in their institutions, and why these variants are important. Nurses who work with providers performing biopsies can identify cases where molecular testing is appropriate based on facility protocols and facilitate early ordering of molecular tests. Nurse navigators should be aware of financial implications related to molecular testing that impact the patient and organization. They play a key role in communicating financial barriers during care planning, identifying financial risk, and connecting patients with financial resources.
Nurse navigators should advocate for standardizing the use of a particular molecular test, participate in evaluating the quality of the test, and use or share resources that facilitate informed consent prior to ordering the test (Table). Standardizing the use of a molecular test reduces confusion associated with determining an appropriate test and empowers pulmonologists, interventional radiologists, or cardiothoracic surgeons who perform biopsies to confidently order the test at time of diagnosis. It also bolsters ease of use in that the care team becomes familiar with a particular test and reference laboratory. Local molecular testing processes must be understood by all members of the multidisciplinary team, and nurse navigators can facilitate the dissemination of practice changes.
Nurse navigators can share data that drive change and advocate for evaluating and improving the way tissue is collected during biopsies or handled by laboratories and communicate the utility of pathologists ordering molecular testing at diagnosis.5,6,38 These actions promote guideline adherence, integration of quality protocols, and streamlined molecular testing processes. This contributes to increased likelihood of meeting benchmark time from diagnosis to treatment and improved patient outcomes.
Conclusion
Through increasing familiarity with molecular testing in NSCLC, collaborating with other members of the multidisciplinary team, and coordinating care for patients in the diagnostic workup phase of NSCLC, nurse navigators play an important role in achieving benchmarks for molecular testing processes and pathologic diagnosis to treatment.
References
- American Cancer Society. Cancer Facts and Figures 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pd
- Martin J. Genetic biomarkers: implications of increased understanding and identification in lung cancer management. Clin J Oncol Nurs. 2020;24:648-656.
- National Cancer Institute. Non-Small Cell Lung Cancer Treatment. 2023. www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq#_4
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Tajarernmuang P, Ofiara L, Beaudoin S, Gonzalez AV. Bronchoscopic tissue yield for advanced molecular testing: are we getting enough? J Thoracc Disc. 2020;12:3287-3295.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version 2.2023. Accessed March 21, 2023. nscl.pdf (nccn.org)
- Reifel JL. Lung Cancer. In: Asch SM, Kerr EA, Hamilton EG, et al (eds). Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. Santa Monica, CA: RAND Corporation; 2000:133-171.
- Vidaver RM, Shershneva MB, Hetzel SJ, et al. Typical time to treatment of patients with lung cancer in a multisite, US-based study. J Oncol Pract. 2016;12:e643-e653.
- Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18:651-659.
- Davies M. Oncogenic-directed therapy for advanced non-small cell lung cancer: implications for the advanced practice nurse. Clin J Oncol Nurs. 2022;26:245-251.
- Owen DH, Singh N, Ismaila N, et al. Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO Living Guideline, Version 2022.2. J Clin Oncol. 2022;41:e10-e20.
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small-cell lung cancer guideline. European Society for Medical Oncology. 2017. https://interactive guidelines.esmo.org/esmo-web-app/gl_toc/index.php?GL_id=60
- Herbst RS, Wu YL, John T, et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41:1830-1840.
- Friend P, Dickman E, Calzone K. Using a genomics taxonomy: facilitating patient care safety and quality in the era of precision oncology. Clin J Oncol Nurs. 2021;25:205-209.
- Germany J, Kueber J. Pharmacogenomic germline testing: applications in oncology nursing. Clin J Oncol Nurs. 2023;27:129-133.
- Thompson JC, Yee SS, Troxel AB, et al. Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNA. Clin Cancer Res. 2016;22:5772-5782.
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142:321-346.
- Mellert H, Foreman T, Jackson L, et al. Development and clinical utility of a blood-based test service for the rapid identification of actionable mutations in non-small cell lung carcinoma. J Mol Diag. 2017;19:404-416.
- Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 2021;16:1647-1662.
- Phallen J, Leal A, Woodward BD, et al. Early noninvasive detection of response to targeted therapy in non-small cell lung cancer. Cancer Res. 2019;79:1204-1213.
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973-1985.
- Postmust PE, Kerr KM, Oudkerk M, et al. Early and Locally Advanced Non-Small Cell Lung Cancer Guideline. European Society for Medical Oncology. 2017. https://interactiveguidelines.esmo.org/esmo-web-app/gl_toc/index.php?GL_id=46
- National Cancer Institute. NCI Dictionary of Genetics Terms. https://www.cancer.gov/publications/dictionaries/genetics-dictionary
- Sheffield BS, Eaton K, Emond B, et al. Cost savings of expedited care with upfront next-generation sequencing testing versus single-gene testing among patients with metastatic non-small cell lung cancer based on current Canadian practices. Curr Oncol. 2023;30:2348-2365.
- Bayot ML, Lopes JE, Naidoo P. Clinical Laboratory. StatPearls. Published 2022. Accessed August 12, 2023. www.ncbi.nlm.nih.gov/books/NBK535358/
- Gardner B, Doose M, Sanchez JI, et al. Distribution of genomic testing resources by oncology practice and rurality: a nationally representative study. JCO Precis Oncol. 2021;5:1060-1068.
- Hollasch M. In-house next-generation sequencing improves turnaround time in NSCLC. OncLive. 2022. In-House Next-Generation Sequencing Improves Turnaround Time in NSCLC (onclive.com)
- Michigan Medicine. Lung Cancer NGS Panel. University of Michigan Laboratories. https://mlabs.umich.edu/tests/lung-cancer-ngs-panel
- Angerilli V, Galuppini F, Pagni F, et al. The role of the pathologist in the next-generation era of tumor molecular characterization. Diagnostics (Basel). 2021;11:339.
- US Food & Drug Administration. Clinical Laboratory Improvement Amendments (CLIA). 2021. Clinical Laboratory Improvement Amendments (CLIA) | FDA
- US Food & Drug Administration. Recently-Approved Devices. 2022. Recently-Approved Devices | FDA
- Centers for Medicare & Medicaid Services. Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer. 2020. NCA - Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer (CAG-00450R) - Decision Memo (cms.gov)
- Grace AL, Trosman JR, Douglas MP, et al. Influence of payer coverage and out-of-pocket costs on ordering of NGS panel tests for hereditary cancer in diverse settings. J Genet Couns. 2022;31:130-139.
- Stanciu J, Tariman JD. Liquid biopsy: a tool for the diagnostic and prognostic evaluation of cancers. Clin J Oncol Nurs. 2020;24:19-21.
- Centers for Medicare & Medicaid Services. Laboratory Date of Service Policy. 2022. www.cms.gov/medicare/medicare-fee-for-service-payment/clini callabfeesched/clinical-lab-dos-policy
- Shah P, Thornton I, Turrin D, Hipskind JE. Informed Consent. StatPearls. 2022. www.ncbi.nlm.nih.gov/books/NBK430827/
- Cappello F, Angerilli V, Munari G, et al. FFPE-based NGS approaches into clinical practice: the limits of glory from a pathologist viewpoint. J Pers Med. 2022;12:750.
- Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non-small cell lung cancer: real-world issues and tough choices. Am Soc Clin Oncol Educ Book. 2019;39:531-542.
- US Department of Health and Human Services. Health Information Privacy. 2022. www.hhs.gov/hipaa/for-professionals/privacy/index.html
- Levy B, Ramkumar B, Morganstein N. Integrating Molecular Testing Results Into Electronic Medical Records. OncLive. 2022. www.onclive.com/view/integrating-molecular-testing-results-into-electronic-medical-records
- Hung H-Y, Tseng Y-H, Chao H-S, et al. Multidisciplinary team discussion results in survival benefit for patients with stage III non-small-cell lung cancer. PLoS One. 2020;15:e0236503.
- Taberna M, Moncayo FG, Jané-Salas E, et al. The multidiscipinary team (MDT) approach and quality of care. Front Oncol. 2020;10:1-16.



